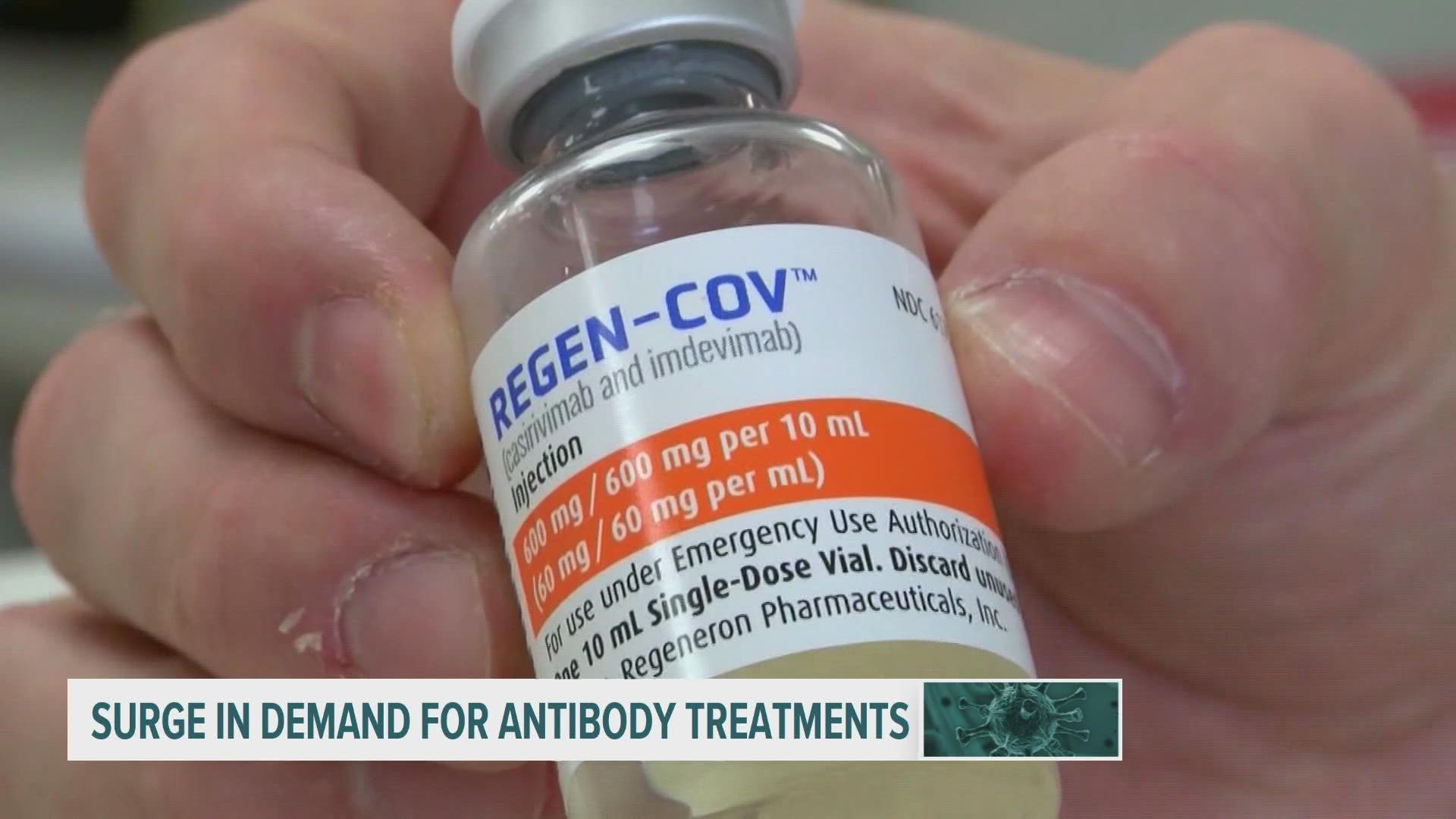
DES MOINES, Iowa — As COVID-19 Delta variant cases rise, monoclonal antibody treatments are gaining popularity, according to the Iowa Department of Public Health.
IDPH said the U.S. Department of Health and Human Services is modifying how these treatments are allocated to states.
As of Sept. 13, health care providers can no longer order the drugs directly from the wholesale company, AmerisourceBergen.
Instead, the federal government determines the number of treatments that will go to each state based on the overall case burden and the state's use of those treatments.
The state health department then identifies and allocates the treatments to sites within the state based on product availability, distribution of high-risk patients and current inventory for providers.
The FDA has granted emergency authorization for three monoclonal antibody treatments: Regeneron, sotrovimab and a joint administration of bamlanivimab and etesevimab.
This week, Iowa received more than 1,800 treatments—552 bamlanivimab and 1,260 Regeneron.
Watch: University of Iowa patients testing Pfizer boosters, Sanofi vaccine
► Download the We Are Iowa app
► Sign up for Local 5's "5 Things to Know" email newsletter
► Subscribe to Local 5 News on YouTube
Health officials urge everyone to continue to practice mitigation strategies to stop the spread of COVID-19:
- Get vaccinated
- Wear a mask as the CDC recommends
- Stay home when you're sick
- Wash your hands frequently
- Practice social distancing