JOHNSTON, Iowa — Iowa will receive its first shipment of 26,000 COVID-19 vaccines around December 13, Gov. Kim Reynolds announced Thursday morning at her press conference.
The first shipment will be Pfizer vaccines, the governor confirmed. Reynolds also noted that the number of vaccines and shipment dates are subject to change. Another shipment of 31,000 Pfizer doses is expected for Dec. 20.
Reynolds also said 54,000 vaccines from Moderna are expected for Dec. 27 as well as another 95,000 doses from Pfizer. Both companies have submitted emergency use authorization with the Food and Drug Administration (FDA).
The Pfizer vaccine has a 95% efficacy against COVID-19, and two doses are required 21 days apart. The Moderna vaccine has a 94.5% efficacy against the virus, and two doses are required 28 days apart.
In total, 172,000 doses are expected for Iowa, meaning 172,000 Iowans will be vaccinated.
The Centers for Disease Control and Prevention (CDC) recommends health care workers and nursing home residents to be the first to get vaccinated, Reynolds said Thursday.
"It's this initial quantity of vaccines that will be prioritized according to the recommendation of the CDC and Iowa will distribute a portion of the allocation to hospitals for their workforce and to our long term care facilities," Reynolds said Thursday.
Reynolds included that the federal government has developed a long-term care pharmacy partnership, which is a program that leverages national pharmacies to help administer the vaccine among long-term care facilities.
"This will allow us to quickly and efficiently vaccine our most vulnerable population first and communities across the state," Reynolds said.
WATCH: Gov. Reynolds' press conference from Thursday
What does the vaccine distribution plan look like?
Iowa Department of Human Services and interim Department of Public Health Director Kelly Garcia discussed the state's vaccine distribution plan during the press conference Thursday.
Storing the vaccines
Garcia noted the differences between storing Pfizer and Moderna vaccines. Pfizer doses require "ultra-cold storage" at negative 70 degrees Celsius. Once thawed, the vaccine is stable at refrigerator temperatures for five days, Garcia explained.
"We've secured 39 locations that can accommodate ultra-cold storage, and we are working on more. Multiple state partners and private businesses have offered support for this function and for that we are tremendously grateful," Garcia said.
The Moderna vaccine requires a more traditional storage temperature of negative 20 degrees Celsius and is stable for refrigerator temperatures for up to 30 days.
Who receives a vaccine first?
Garcia said critical populations will be prioritized to get the vaccine first.
"We have a significant number of populations that fall into this priority category, so some prioritization within this category sub prioritization is necessary," Garcia noted.
Health care professionals at hospitals will be prioritized, but the state will hone in on long-term care residents and their direct care staff, Garcia said. The partnership between pharmacies and long-term care facilities will be critical in distributing the vaccine.
"Part of the challenge and vaccinating this population is that their workforce is already at capacity responding to the pandemic. The federal government created this pharmacy partnership program, which leverages national pharmacy chains to assist in administering the vaccine to our long term care facilities," Garcia explained.
CVS, Walgreens and Community Pharmacy will be participating in the program. It'll also ensure vaccine access in rural areas of Iowa.
Garcia estimated more populations will be able to receive the vaccine by June 2021.
The vaccine is not approved for children, therefore it is not required for school.
What's the timeline for distribution?
Again, the number of doses and shipping dates are subject to change. Here is a look at the first three weeks of vaccine distribution:

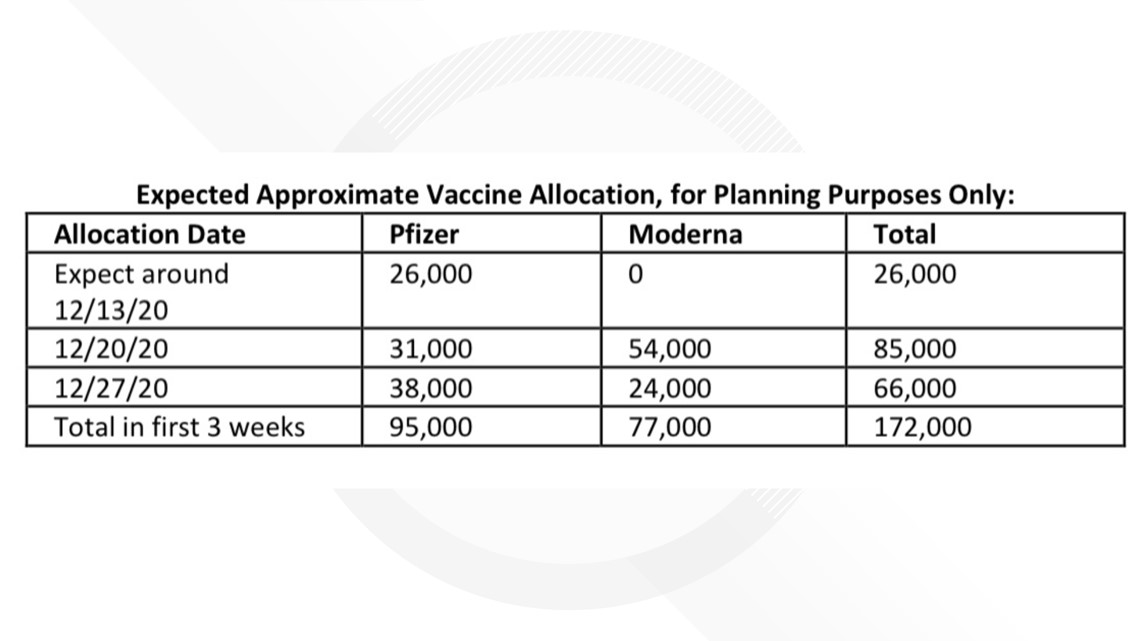
The first shipment of 26,000 Pfizer vaccines are expected for Dec. 13. A portion of these doses will be distributed to six health care sites in major metropolitan areas, Garcia said. The state will not post locations of vaccines for security purposes.
On Dec. 20, the state will receive it's second allocation of Pfizer vaccines and its first allocation of Moderna vaccines. Garcia said vaccines will be sent to long-term care facilities during this week via the pharmacy partnership program.
On Dec. 27, Iowa is expected to accept additional allocations of Pfizer and Moderna doses.
Both Pfizer and Moderna require two doses for the vaccine.
The Infectious Disease Advisory Council (IDAC) will assist the state in developing COVID-19 vaccine prioritization of populations for early states of vaccine response when supply is limited, Garcia said. This group will bring together ethical and clinical expertise from across the state, representing multiple perspectives.
"The reason for this is to minimize health inequities based on geography, poverty, and other social determinants. This group will also provide input as additional vaccines become available, and will help us navigate our distribution of therapeutics," Garcia said.
More updates on vaccine distribution will be provided as details and federal guidance "are solidified and confirmed," said Garcia.
Is the vaccine safe?
Gov. Reynolds brought on Dr. Brooks Jackson, Vice President of Medical Affairs at University of Iowa Hospitals and Clinics, to discuss the vaccine and how it works.
Jackson was directly involved in COVID-19 vaccine trials, Reynolds said. Jackson said Iowans should feel confident with vaccines due to the efficacy and safety data reported.
"The COVID vaccines like all drugs and medical devices are going through multiple phases of rigorous testing analysis and review as they are developed," Jackson said.
"In fact, the UI Carver College of Medicine was one of the study sites for the Pfizer vaccine trial, enrolling 270 volunteers for the clinical study, including myself," Jackson said.
"I was one of the first to enrolling in July, although I don't know whether I received the placebo or the vaccine, but I am very excited and feel very confident that this will be a very efficacious and safe vaccine and the Moderna one looks very similar," concluded Jackson.
Jackson acknowledged that the development of these vaccines have been quick, but said there's no need to worry since so many people participated in the study for the Pfizer vaccine.
"The safety from these large trials will be available in the next one to two weeks. But the safety data from the phase one two studies, and our own experience with the Pfizer vaccine trial and Carver College of Medicine is very reassuring," Jackson said.
While a vaccine is promising, Jackson said Iowans need to continue other mitigation efforts while they wait for their own dose:
- Stay home when sick
- Wear a mask
- Wash hands frequently
- Practice social distancing
- Avoid traveling
"The weeks and months ahead look indeed very promising but we cannot let down our guard just yet. Let's be cautiously optimistic if we stay vigilant and work together. I think we will likely be able to look forward to, like, returning to normal sometime in 2021," Jackson said.


