Iowa is now on the verge of distributing the Pfizer coronavirus vaccine following the Food and Drug Administration approving it for emergency use Friday night.
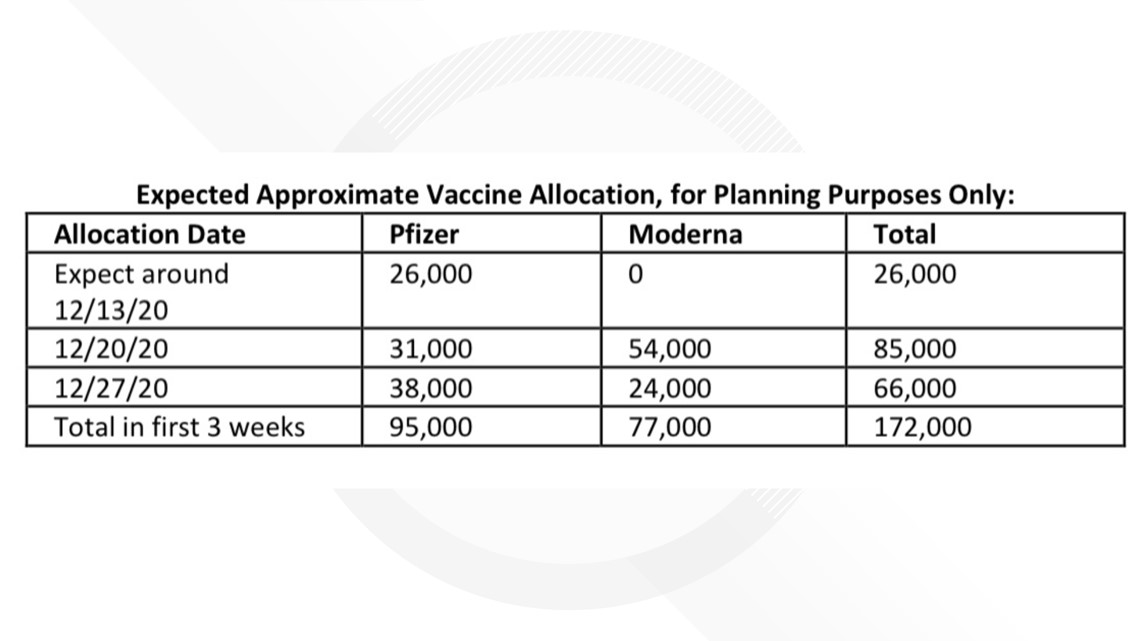
Gov. Kim Reynolds said earlier this month that her administration expected to receive a shipment of 26,000 around Dec. 13.
31,000 more of the Pfizer vaccine should be in the state by Dec. 20.
54,000 vaccines from Moderna are expected for Dec. 27 as well as another 95,000 doses from Pfizer.
Moderna is still awaiting emergency use authorization from the FDA.
In total, the state of Iowa anticipates getting roughly 172,000 doses by the end of the year.
The initial vaccines will be used as the first dose of a two-dose series.
IDPH Interim Director Kelly Garcia said Wednesday the federal government reassured them there will be enough vaccine supply, and that the state will receive shipments in time for people to get their second vaccination.

In a release Friday night, the Iowa Department of Public Health said the state will begin receiving the vaccines the week of Dec. 13.
Health care workers and long-term care (LTC) residents will be part of the first phase of vaccinations.
“While this is a positive step forward, it will take time until the vaccine is widely available," Reynolds said in a statement. "Until then, we must continue to mitigate the virus by practicing public health measures so we can protect the most vulnerable and preserve hospital resources while keeping our economy open and our kids in school."
The Pfizer vaccine has a 95% efficacy against COVID-19 and two doses are required 21 days apart.
The Moderna vaccine has a 94.5% efficacy against the virus, and two doses are required 28 days apart.
Walgreens, CVS and Community Pharmacy locations will administer the vaccines free of charge to LTC residents in the following fashion:
- Schedule and coordinate on-site clinic date(s) directly with each facility.
- Coordinate a series of visits to administer both doses of vaccine and vaccinate any new residents and staff.
- Order vaccines and associated supplies (e.g., syringes, needles, personal protective equipment).
Around 1,500 providers have signed up to administer the vaccine with approximately 20-40 new applications submitted to the state each day, according to the release.
“While we anticipated this approval, it is nonetheless great news for Iowans. The vaccine will allow us to move forward in our fight against COVID-19," Garcia said in a statement. "I am incredibly grateful to our team and our partners across the state who have spent countless hours planning for the deployment of this vaccine."
Additional information from IDPH:
COVID-19 vaccines will continue to increase in supply, ensuring that by mid-2021, anyone who wants to receive the vaccine will have the opportunity to do so. IDPH has established a healthcare provider COVID-19 Vaccine Call Center. To reach the COVID-19 Vaccine Call Center, call 1-800-831-6293, Option 1.
The FDA will hold a press conference at 8 a.m. Saturday to discuss the latest vaccine developments.
The FDA released fact sheets on the Pfizer vaccine for patients planning to get vaccinated and health care providers administering the vaccine.
In the fact sheet, it states people who have had severe allergic reactions after a previous dose of the Pfizer vaccine or people who have had severe allergic reactions to any ingredient in the vaccine should not get it.
The FDA also noted reports of severe allergic reactions during mass vaccinations outside of clinical trials.
University of Iowa Health Care statement:
Now that the Food and Drug Administration (FDA) has approved the Pfizer-BioNTech COVID-19 vaccine for emergency use, University of Iowa Health Care is excited to be one milestone closer to implementing its detailed vaccination plans, first for its employees. Health care workers have been identified nationally and by the state to be among the first to be vaccinated.
UI Health Care has been preparing for months for the unique storage and administration details required for this particular vaccine and is one of six health care sites in Iowa that will receive the state’s first limited number of doses. The first vaccinations are expected as early as next week, following final guidance on vaccine prioritization and distribution from the CDC’s Advisory Committee on Immunization Practices (ACIP).
We’re proud to have participated in the Pfizer-BioNTech clinical trial here within UI Health Care,” says Brooks Jackson, MD, MBA, University of Iowa Vice President for Medical Affairs and the Tyrone D. Artz Dean of the UI Carver College of Medicine. “We believe that this vaccine is safe and will be effective in preventing COVID-19. While supply of the vaccine will be initially limited, we will offer the COVID-19 vaccine to all UI Health Care employees who would like to receive it.
More on the coronavirus vaccine
- COVID-19 vaccine questions answered
- VERIFY: You can donate blood, but not plasma, after getting COVID-19 vaccine
- Fauci: US near normal by end of 2021 if 75-80% get coronavirus vaccine
- Rural pharmacies prepare to deliver COVID-19 vaccines
- Hy-Vee to hire 1,000 workers for COVID-19 vaccine distribution
- University of Iowa Hospitals & Clinics expect to start vaccinating staff next week
- 'That's a scam': You can't pay for early access to COVID-19 vaccine, regulators warn