DES MOINES, Iowa — Since America seemingly shut down because of the COVID-19 pandemic, scientists raced to get a safe and effective vaccine.
Now that a vaccine has been approved for emergency use, some people are hesitant, and even not willing, to get it.
Katie Adrian, who lives in Vinton, said she’s choosing to not get Pfizer’s coronavirus vaccine.
"Personally, speaking for myself, I will not be receiving this vaccination ever,” Adrian said. “I don't believe that there are enough benefits to it to outweigh the risks that it would entail."
Adrian also said she doesn’t feel it has been studied enough.
While the U.S. Federal Drug Administration’s emergency approval of the Pfizer vaccine happened in record time, Dr. Patricia Winokur, principal investigator for the University of Iowa’s Pfizer clinical trial site, said researchers did their due diligence.
"The approvals for a lot of drugs, we usually look at about 3,000 individuals in phase three trials,” Winokur said. “For Pfizer’s, we had 44,000 people, half of whom got the active drug."
Not all doctors feel vaccination is the best route.
Dr. Lee Merritt, an orthopedic surgeon, said the medical field should be more focused on exploring alternative treatments.
"We've maybe made some assumptions in the past about vaccines being the be-all, end-all of how we get through the world,” Merritt said.
Merritt said she strongly believes hydroxychloroquine is one of the best treatment options for COVID-19, and said doctors in America should be prescribing it.
This week, there were reports the American Medical Association (AMA) changed its stance on prescribing hydroxychloroquine to treat COVID-19.
The AMA said Wednesday they have not.
“In March, AMA urged caution about prescribing hydroxychloroquine off-label to treat COVID19,” the AMA said in a tweet. “Our position remains unchanged.”
According to the world's leading scientists and health officials, vaccination stops the spread of viruses. Medical experts said they prevent people from ever getting sick and needing those alternate treatment methods.
"We've got to get people vaccinated as quickly and as expeditiously as we possibly can until we get that herd immunity, as we say, which will require, in my opinion, about 75% to 80% of the population getting vaccinated,” Dr. Anthony Fauci said on Good Morning America Tuesday.
Without immunities, the Centers for Disease Control and Prevention (CDC) reports viruses can spread rapidly if an infected person is around a lot of unvaccinated people.
That changes, however, if the infected person is around mostly vaccinated people since the virus won’t have a lot of opportunities to spread.

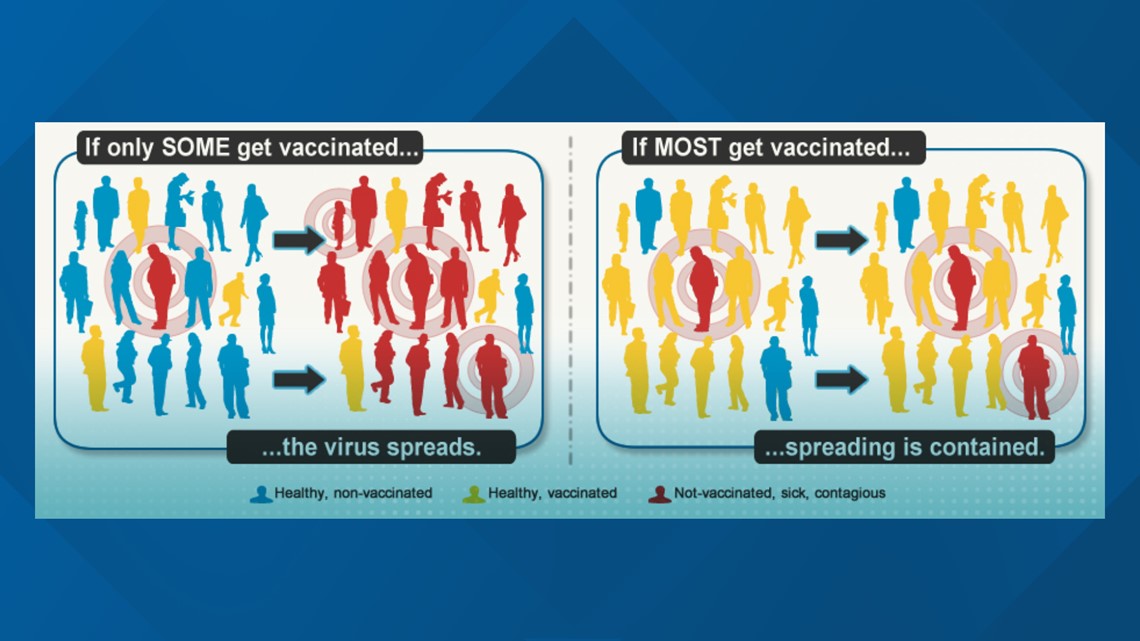
"Getting enough of the population vaccinated to provide a so-called herd immunity or herd protection, is going to take a while," said Dr. Ravi Vemuri, medical director of infectious diseases at MercyOne.
It will also take getting a lot more people on board to get the shot.
But that's something Adrian said she won’t do unless there are more studies and vaccine manufacturers can be held liable for potential injuries.
"No product will ever be completely safe for everyone,” Adrian said. “There are always rare exceptions and circumstances, but for there to be the removal of full liability is really concerning from a safety standpoint."
The FDA released fact sheets on the Pfizer vaccine for patients planning to get vaccinated and health care providers administering the vaccine.
In the fact sheet, it states people who have had severe allergic reactions after a previous dose of the Pfizer vaccine or people who have had severe allergic reactions to any ingredient in the vaccine should not get it.
The FDA also noted reports of severe allergic reactions during mass vaccinations outside of clinical trials.